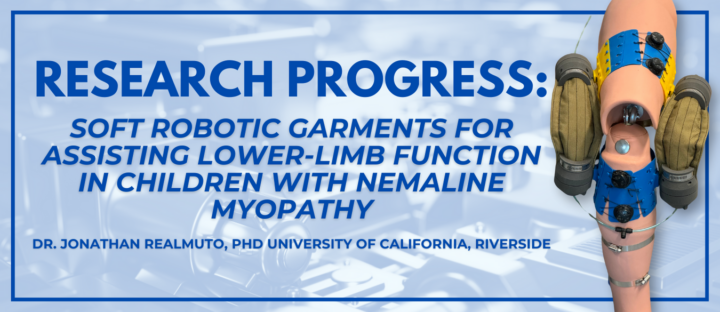
At AFBS, we’re committed to finding treatments for Nemaline Myopathy (NM) through a diverse array of research approaches. From gene therapy to small molecules and disease management strategies, we’re investing in initiatives that hold the most promise for our community and we’re thrilled to share an exciting update on a groundbreaking Nemaline Myopathy exosuit research…
Read MoreRunning for Kinsley: Erica Redinbo is Putting in the Miles for a Brighter Future for Those with Nemaline Myopathy
The AFBS community is filled with inspiring stories of resilience and strength. Today, we shine a light on Erica Redinbo, a mother running the 2024 Boston Marathon in honor of her daughter Kinsley, who bravely battled Nemaline Myopathy (NM). Erica describes Kinsley as the “strongest person I’ve ever known,” even though NM took her physical…
Read MoreBuilding Strength: Promising Developments in Gene Therapy for Nemaline Myopathy
There’s been exciting progress on a new approach treating Nemaline Myopathy (NM), the rare muscle disorder that causes weakness and low muscle tone. While researchers have identified twelve different faulty genes that cause NM and prevent muscles from working properly (ACTA1, NEB, TPM2, TPM3, KBTBD13, CFL2, KLHL40, KLHL41, LMOD3, MYPN, TNNT1, and TNNT3, with the…
Read MoreRegister with the CMDIR: Leading the Way to Treatments Begins with You!
Living with Nemaline Myopathy (NM)? Join the global fight for treatments & therapies! Register with the CMDIR and unlock access to clinical trials, resources, and a supportive community. Be the hero of your story.
Read MoreAFBS Year in Review 2023: Connecting, Empowering, and Advancing Research for Nemaline Myopathy
2023 was a landmark year for the AFBS community, marked by unwavering dedication, meaningful connections, and significant progress in the search for treatments for Nemaline Myopathy (NM). Our Year in Review video is a powerful testament to the collective strength and passion driving this journey.
Read MoreCommunity Spotlight: Audrey
This “Community Spotlight” series highlights and celebrates our unique and wonderful NM community members. The Q&A will give a peek into the lives of individuals living with NM and the diversity they represent.
Read MoreAmplifying Voices, Building Strength: AFBS’ Webinar Series Culmination
The journey to find treatments for rare congenital muscle disease, Nemaline Myopathy, is a collective effort, and A Foundation Building Strength (AFBS) has been at the forefront of this mission. Recently, the organization concluded a groundbreaking 10-part webinar series titled “Amplifying the NM Community’s Voice,” made possible by the Patient-Centered Outcomes Research Institute (PCORI). This…
Read MoreRunning for Hope: AFBS at the 2023 TCS NYC Marathon
The 2023 TCS NYC Marathon was more than just a race; it was a powerful demonstration of collective strength, determination, and unwavering support for those affected by Nemaline Myopathy (NM). A Foundation Building Strength (AFBS) spearheaded a team of remarkable individuals who not only laced up their running shoes but also rallied communities and hearts…
Read MoreAFBS Attends the 28th Annual International World Muscle Society Congress in South Carolina
Recently, the AFBS team had the privilege of attending the World Muscle Society Congress in South Carolina, where AFBS’ Scientific Director, Gus Dziewczapolski, AFBS’ Program Manager, Stacy Cossette, and AFBS’ PCORI Program Coordinator, Sarah Foye, participated in this enlightening event. Pictured Above: AFBS Scientific Director, Gus Dziewczapolski, AFBS Scientific Advisory Dr. Alan Beggs, AFBS Program Director Stacy…
Read MoreThe Road to Treatment: Preparing the Nemaline Myopathy Community for Clinical Trials
Amplifying the NM Community’s Voice” Webinar Recap In our recent webinar, we delved into how you can play a pivotal role in shaping the future of Nemaline Myopathy treatments, underscoring the essential role the community plays in accelerating treatment timelines. We were honored to be joined by exceptional guest speakers, including Jill Anne Castle, a…
Read MoreIntroducing Nemaline Myopathy Resource Kit and Awareness Card: Empowering the NM Community
At A Foundation Building Strength (AFBS), our unwavering commitment to those affected by Nemaline Myopathy (NM) drives us to continually develop resources that empower individuals and families on their journey. We are excited to introduce two new resources that aim to provide valuable information, support, and awareness about NM: the Nemaline Myopathy Resource Kit and…
Read More